Dilated Cardiomyopathy Market Witnesses Robust Growth During the Study Period (2020–2034) Amid Advancements in Cardiovascular Therapies | DelveInsight
The dilated cardiomyopathy market is experiencing steady growth, driven by rising prevalence of heart failure, improved diagnostic techniques, and advancements in genetic testing. Increasing R&D investment in gene therapies and novel drug candidates is expanding treatment options. Additionally, greater awareness and screening initiatives are supporting earlier diagnosis and management.
New York, USA, June 05, 2025 (GLOBE NEWSWIRE) -- Dilated Cardiomyopathy Market Witnesses Robust Growth During the Study Period (2020–2034) Amid Advancements in Cardiovascular Therapies | DelveInsight
The dilated cardiomyopathy market is experiencing steady growth, driven by rising prevalence of heart failure, improved diagnostic techniques, and advancements in genetic testing. Increasing R&D investment in gene therapies and novel drug candidates is expanding treatment options. Additionally, greater awareness and screening initiatives are supporting earlier diagnosis and management.
DelveInsight’s Dilated Cardiomyopathy Market Insights report includes a comprehensive understanding of current treatment practices, emerging dilated cardiomyopathy drugs, market share of individual therapies, and current and forecasted dilated cardiomyopathy market size from 2020 to 2034, segmented into 7MM [the United States, the EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan].
Key Takeaways from the Dilated Cardiomyopathy Market Report
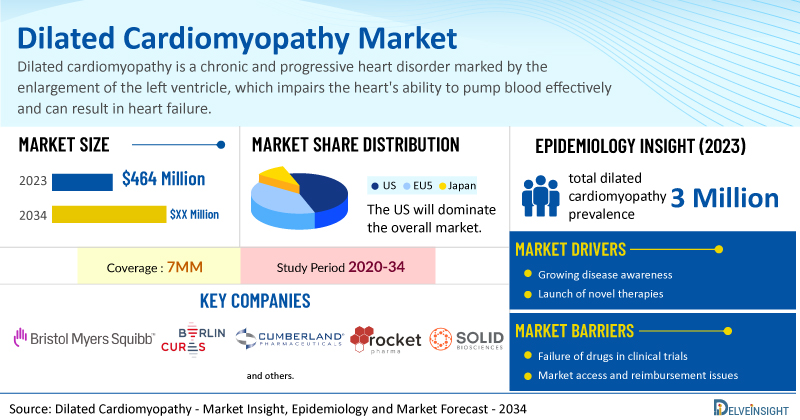
- According to DelveInsight’s analysis, the total dilated cardiomyopathy market size in the 7MM was estimated to be nearly USD 464 million in 2023, and it is expected to grow positively by 2034.
- Amgen’s CORLANOR (ivabradine) generated the second-highest revenue of USD 90.3 million in the US in 2023.
- In the 7MM, the total dilated cardiomyopathy prevalence was estimated to be approximately 3 million in 2023, of which the US accounted for around 45%.
- Prominent companies, including Bristol-Myers Squibb, Berlin Cures, Cumberland Pharmaceutical, Rocket Pharmaceutical, Solid Bioscience, and others, are actively working on innovative dilated cardiomyopathy drugs. These novel dilated cardiomyopathy therapies are anticipated to enter the dilated cardiomyopathy market in the forecast period and are expected to change the market.
- Some of the key dilated cardiomyopathy treatments include Danicamtiv (MYK-491), BC007, DYSCORBAN (ifetroban), REN-001, AVB-401, SGT-601, and others.
Discover which new treatment for dilated cardiomyopathy is expected to grab the market share @ Dilated Cardiomyopathy Market Report
Dilated Cardiomyopathy Overview
Dilated cardiomyopathy is a chronic and progressive heart disorder marked by the enlargement of the left ventricle, which impairs the heart's ability to pump blood effectively and can result in heart failure. By definition, patients exhibit systolic dysfunction, though they may or may not show clear signs of heart failure.
The clinical presentation can vary widely from no symptoms to advanced heart failure. Common symptoms include shortness of breath during exertion, fatigue, palpitations, general dyspnea, swelling in the limbs, and arrhythmias such as ventricular tachycardia and atrial fibrillation.
While many cases are idiopathic, some result from external factors such as viral infections (e.g., myocarditis), exposure to toxins (like excessive alcohol or chemotherapy drugs), autoimmune conditions, or nutritional deficiencies. Approximately 50% of cases have a genetic or familial basis.
Diagnosing dilated cardiomyopathy involves a structured process to detect abnormalities in heart structure and function and to exclude other possible causes of cardiac impairment. This typically includes a thorough clinical evaluation, imaging techniques, lab investigations, and genetic testing.
Dilated Cardiomyopathy Epidemiology Segmentation
The dilated cardiomyopathy epidemiology section provides insights into the historical and current dilated cardiomyopathy patient pool and forecasted trends for the 7MM. It helps recognize the causes of current and forecasted patient trends by exploring numerous studies and views of key opinion leaders.
The dilated cardiomyopathy market report proffers epidemiological analysis for the study period 2020–2034 in the 7MM segmented into:
- Total Prevalent Cases of Dilated Cardiomyopathy
- Total Diagnosed Prevalent Cases of Dilated Cardiomyopathy
- Gender-specific Cases of Dilated Cardiomyopathy
- Familial and Non-familial Cases of Dilated Cardiomyopathy
Download the report to understand which factors are driving dilated cardiomyopathy epidemiology trends @ Dilated Cardiomyopathy Treatment Options
Dilated Cardiomyopathy Treatment Market
Currently, there is no cure for dilated cardiomyopathy, but various treatments are available to manage symptoms and reduce the risk of complications. The choice of treatment depends on the extent of the heart’s dysfunction and the patient’s specific symptoms. The primary goals of treating cardiomyopathy are to:
- Prevent the condition from worsening,
- Address and manage any underlying or contributing health issues, and
- Minimize complications, including the risk of sudden cardiac arrest (SCA), with the ultimate aim of helping patients maintain a normal quality of life.
Pharmacological interventions commonly used include diuretics, inotropic agents, afterload reducers, beta-blockers, anticoagulants, and anti-arrhythmic drugs. Inotropic agents used in treatment include digoxin, dobutamine, dopamine, epinephrine, norepinephrine, vasopressin, and milrinone. Afterload-reducing drugs include ACE inhibitors such as captopril, enalapril, lisinopril, and fosinopril, as well as angiotensin receptor blockers (ARBs) like losartan. Milrinone is notable for its dual action as an inotropic agent and vasodilator.
ACE inhibitors and ARBs have shown effectiveness in managing heart failure with reduced ejection fraction and are recommended for patients with DCM. Stronger anticoagulants, including warfarin, heparin, and enoxaparin, require careful dosage adjustments and regular blood monitoring.
Amgen’s CORLANOR (ivabradine), approved by the US FDA, is currently the only dilated cardiomyopathy treatment drug in its class in the U.S. It works by selectively inhibiting the If current in the sinoatrial node, thereby slowing heart rate without affecting myocardial contractility or ventricular repolarization. In April 2019, the FDA approved CORLANOR for treating stable, symptomatic heart failure due to DCM in pediatric patients aged 6 months to 18 years.
Dilated cardiomyopathy is a progressive disorder marked by continual myocardial injury, structural changes, and declining cardiac function. Hence, there is an urgent need for new treatments for dilated cardiomyopathy that can halt or delay disease progression, prevent adverse cardiac remodeling, and preserve heart function over time.
Learn more about the dilated cardiomyopathy treatment options @ Dilated Cardiomyopathy Treatment Guidelines
Dilated Cardiomyopathy Emerging Drugs and Companies
Several novel therapies are under development, including Bristol-Myers Squibb’s Danicamtiv (MYK-491), Berlin Cures’ BC007, Cumberland Pharmaceuticals’ DYSCORBAN (ifetroban), Rocket Pharmaceuticals’ REN-001, Solid Biosciences’ AVB-401 and SGT-601, among others.
Danicamtiv (MYK-491), developed by Bristol Myers Squibb, is an orally available small molecule aimed at enhancing cardiac contractility. It works by increasing the formation of myosin-actin cross-bridges during heart muscle contraction, while maintaining diastolic function. Since myosin is the motor protein responsible for contracting the heart muscle by interacting with actin, MYK-491 targets the fundamental biomechanical dysfunction seen in patients with systolic heart failure and dilated cardiomyopathy. In these conditions, the left ventricle is too weakened and enlarged to pump blood efficiently. By boosting myosin-actin interactions, MYK-491 seeks to improve cardiac performance.
BC007, developed by Berlin Cures, is a DNA aptamer that neutralizes harmful autoantibodies targeting the beta-1 adrenoceptor—a key G-protein coupled receptor involved in regulating heart rate and contractile force. Delivered intravenously, BC007 is part of the company’s APTACURES platform, which focuses on aptamers that bind to autoantibodies against GPCRs.
Ifetroban, the active component of DYSCORBAN from Cumberland Pharmaceuticals, is a highly selective thromboxane receptor (TPr) antagonist. It is designed to block fibrotic and inflammatory pathways in the heart, especially those initiated by the absence of dystrophin protein. By inhibiting key molecular signals involved in inflammation and tissue scarring, ifetroban may help preserve cardiac function in patients at risk of fibrotic damage.
The anticipated launch of these emerging dilated cardiomyopathy drugs are poised to transform the dilated cardiomyopathy market landscape in the coming years. As these cutting-edge therapies continue to mature and gain regulatory approval, they are expected to reshape the dilated cardiomyopathy market landscape, offering new standards of care and unlocking opportunities for medical innovation and economic growth.
To know more about dilated cardiomyopathy clinical trials, visit @ Dilated Cardiomyopathy Pipeline Market
Dilated Cardiomyopathy Market Dynamics
The dilated cardiomyopathy market dynamics are anticipated to change in the coming years. The increasing prevalence of drug or alcohol abuse, cardiac muscle infections, and rising public awareness in the 7MM are anticipated to fuel the growth of the dilated cardiomyopathy market, supported by advances in device therapies such as ICD, CRT, and surgical interventions that significantly reduce mortality and hospitalization rates; furthermore, opportunities exist to enhance genetic screening for at-risk individuals, improve disease management, and develop novel therapies with better efficacy, duration of action, safety profiles, and biomarker identification.
Furthermore, many potential therapies are being investigated for the treatment of dilated cardiomyopathy, and it is safe to predict that the treatment space will significantly impact the dilated cardiomyopathy market during the forecast period. Moreover, the anticipated introduction of emerging therapies with improved efficacy and a further improvement in the diagnosis rate is expected to drive the growth of the dilated cardiomyopathy market in the 7MM.
However, several factors may impede the growth of the dilated cardiomyopathy market. Dilated cardiomyopathy is a heterogeneous condition with various underlying etiologies, clinical manifestations, and prognostic factors, making it difficult to develop standardized treatment approaches; it is challenging to diagnose due to its nonspecific symptoms and variable presentation, often leading to delays in diagnosis and treatment initiation, and despite advancements in therapy, curative options remain lacking, existing treatments primarily aim to alleviate symptoms and slow progression, while the condition is associated with a poor long-term prognosis, with survival rates below 50% at 10 years and many patients facing progressive heart failure, arrhythmias, and sudden cardiac death.
Moreover, dilated cardiomyopathy treatment poses a significant economic burden and disrupts patients’ overall well-being and QOL. Furthermore, the dilated cardiomyopathy market growth may be offset by failures and discontinuation of emerging therapies, unaffordable pricing, market access and reimbursement issues, and a shortage of healthcare specialists. In addition, the undiagnosed, unreported cases and the unawareness about the disease may also impact the dilated cardiomyopathy market growth.
| Dilated Cardiomyopathy Report Metrics | Details |
| Study Period | 2020–2034 |
| Dilated Cardiomyopathy Report Coverage | 7MM [The United States, the EU-4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan] |
| Dilated Cardiomyopathy Market Size in 2023 | USD 464 Million |
| Key Dilated Cardiomyopathy Companies | Bristol-Myers Squibb, Berlin Cures, Cumberland Pharmaceutical, Rocket Pharmaceutical, Solid Bioscience, and others |
| Key Dilated Cardiomyopathy Therapies | Danicamtiv (MYK-491), BC007, DYSCORBAN (ifetroban), REN-001, AVB-401, SGT-601, and others |
Scope of the Dilated Cardiomyopathy Market Report
- Dilated Cardiomyopathy Therapeutic Assessment: Dilated Cardiomyopathy current marketed and emerging therapies
- Dilated Cardiomyopathy Market Dynamics: Conjoint Analysis of Emerging Dilated Cardiomyopathy Drugs
- Competitive Intelligence Analysis: SWOT analysis and Market entry strategies
- Unmet Needs, KOL’s views, Analyst’s views, Dilated Cardiomyopathy Market Access and Reimbursement
Discover more about dilated cardiomyopathy treatment drugs in development @ Dilated Cardiomyopathy Clinical Trials
Table of Contents
| 1. | Dilated Cardiomyopathy Market Key Insights |
| 2. | Dilated Cardiomyopathy Market Report Introduction |
| 3. | Dilated Cardiomyopathy Market Overview at a Glance |
| 4. | Dilated Cardiomyopathy Market Executive Summary |
| 5. | Disease Background and Overview |
| 6. | Dilated Cardiomyopathy Treatment and Management |
| 7. | Dilated Cardiomyopathy Epidemiology and Patient Population |
| 8. | Patient Journey |
| 9. | Dilated Cardiomyopathy Marketed Drugs |
| 10. | Dilated Cardiomyopathy Emerging Drugs |
| 11. | Seven Major Dilated Cardiomyopathy Market Analysis |
| 12. | Dilated Cardiomyopathy Market Outlook |
| 13. | Potential of Current and Emerging Therapies |
| 14. | KOL Views |
| 15. | Unmet Needs |
| 16. | SWOT Analysis |
| 17. | Appendix |
| 18. | DelveInsight Capabilities |
| 19. | Disclaimer |
| 20. | About DelveInsight |
Related Reports
Dilated Cardiomyopathy Pipeline
Dilated Cardiomyopathy Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key dilated cardiomyopathy companies, including Berlin Cures, AstraZeneca, Biohaven Pharmaceuticals, Tenaya Therapeutics, among others.
Dilated Cardiomyopathy Epidemiology Forecast
Dilated Cardiomyopathy Epidemiology Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted dilated cardiomyopathy epidemiology in the 7MM, i.e., the United States, EU5 (Germany, Spain, Italy, France, and the United Kingdom), and Japan.
Hypertrophic Cardiomyopathy Market
Hypertrophic Cardiomyopathy Market Insights, Epidemiology, and Market Forecast – 2034 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key hypertrophic cardiomyopathy companies including Bristol Myers Squibb, Cytokinetics, Novartis, Imbria Pharmaceuticals, Univar Solutions B.V., among others.
Hypertrophic Cardiomyopathy Pipeline
Hypertrophic Cardiomyopathy Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key hypertrophic cardiomyopathy companies, including Cytokinetics, Imbria, Bristol-Myers Squibb, Tenaya Therapeutics, Lexeo Therapeutics, Inc., BioMarin, among others.
Restrictive Cardiomyopathy Market
Restrictive Cardiomyopathy Market Insights, Epidemiology, and Market Forecast – 2034 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key restrictive cardiomyopathy companies including GlaxSoSmithKline, Teva Pharmaceuticals, Cipla, Sun Pharmaceuticals, among others.
DelveInsight’s Pharma Competitive Intelligence Service: Through its CI solutions, DelveInsight provides its clients with real-time and actionable intelligence on their competitors and markets of interest to keep them stay ahead of the competition by providing insights into the latest therapeutic area-specific/indication-specific market trends, in emerging drugs, and competitive strategies. These services are tailored to the specific needs of each client and are delivered through a combination of reports, dashboards, and interactive presentations, enabling clients to make informed decisions, mitigate risks, and identify opportunities for growth and expansion.
Other Business Pharmaceutical Consulting Services
Healthcare Conference Coverage
Discover how a mid-pharma client gained a level of confidence in their soon-to-be partner for manufacturing their therapeutics by downloading our Due Diligence Case Study
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance. Get hassle-free access to all the healthcare and pharma market research reports through our subscription-based platform PharmDelve.
Connect with us on LinkedIn|Facebook|Twitter

Contact Us Shruti Thakur info@delveinsight.com +14699457679 www.delveinsight.com
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
